

FOB Price : Get a Price/Quote
Min.Order :
Certification : CE
Brand Name : GENEDIAN
Payment Terms : T/T
brand name : GENEDIAN
certification : CE
min.order :
warranty :
payment terms : T/T
Packaging :
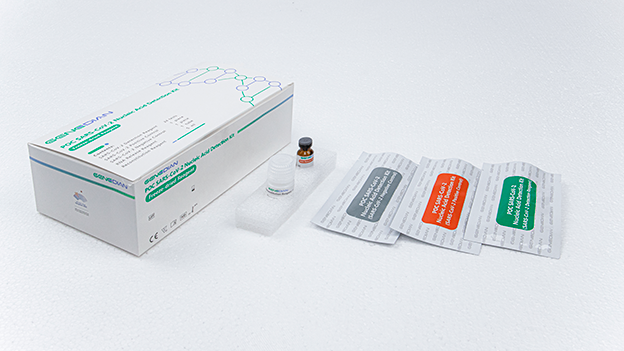
Specification : 24 tests/kit
place of origin : Zhejiang




 Ordinary
Ordinary
 verified
verified
Business Type Manufacturer
Country / Region Zhejiang,China
Main Products Chinese Medical Instruments, In vitro Diagnosis Reagents, Immune Diagnostic Reagents, POCT Diagnostic Reagents, Digital Imaging, Quality Assurance & Safety Control, Laboratory Instruments, Data Analytics, IT Software, Medical Design & Manufacturing Products
Main Markets
Brand : GENEDIAN
Min.Order :

Brand : GENEDIAN
Min.Order : Vial(s)

Brand : GENEDIAN
Min.Order :

Brand : GENEDIAN
Min.Order :

Brand : GENEDIAN
Min.Order :
brand name : GENEDIAN
certification :
fob price :
min.order :
warranty :
payment terms : T/T
Packaing :
Specification : 24 tests/kit
Trademark :
Production Capacity :
place of origin : Zhejiang
Manag Certifica : CE