



FOB Price : Get a Price/Quote
Min.Order : 1000 Piece(s)
Certification : CE
Brand Name : TWIZUK
Payment Terms : T/T
brand name : TWIZUK
certification : CE
min.order : 1000 Piece(s)
warranty : \
payment terms : T/T
Packaging : 1 Test/Box, 5Tests/Box, 25Tests/Box
Specification : \
place of origin : Zhejiang
Clinical sample |
COVID-19 Coronavirus Real Time PCR Kit (RT-PCR) | Total |
||
| Positive | Negative | |||
| SARS-CoV-2/Influenza A+B Antigen Detection Kit (N line) (Colloidal Gold Method) |
Positive | 145 | 0 | 145 |
| Negative | 3 | 517 | 520 | |
| Total | 148 | 517 | 665 | |
Clinical sample |
cobas® Liat® Influenza A/B & RSV Assay(RT-PCR) | Total |
||
| Positive | Negative | |||
| SARS-CoV-2/Influenza A+B Antigen Detection Kit (A line) |
Positive | 155 | 0 | 155 |
| Negative | 4 | 506 | 510 | |
| Total | 159 | 506 | 665 | |
Clinical sample |
cobas® Liat® Influenza A/B & RSV Assay(RT-PCR) | Total |
||
| Positive | Negative | |||
| SARS-CoV-2/Influenza A+B Antigen Detection Kit (B line) |
Positive | 144 | 1 | 145 |
| Negative | 5 | 515 | 520 | |
| Total | 149 | 516 | 665 | |




 Ordinary
Ordinary
 verified
verified
Business Type Manufacturer
Country / Region Zhejiang,China
Main Products General Examination Instruments, Disposable medical consumables, In vitro Diagnosis Consumables, In vitro Diagnosis Reagents, Blood Diagnosis Reagents, Immune Diagnostic Reagents, Biochemical Diagnostic Reagents, POCT Diagnostic Reagents, Medical Design & Manufacturing Products, Others
Main Markets EasternEurope,EasternAsia,WesternEurope
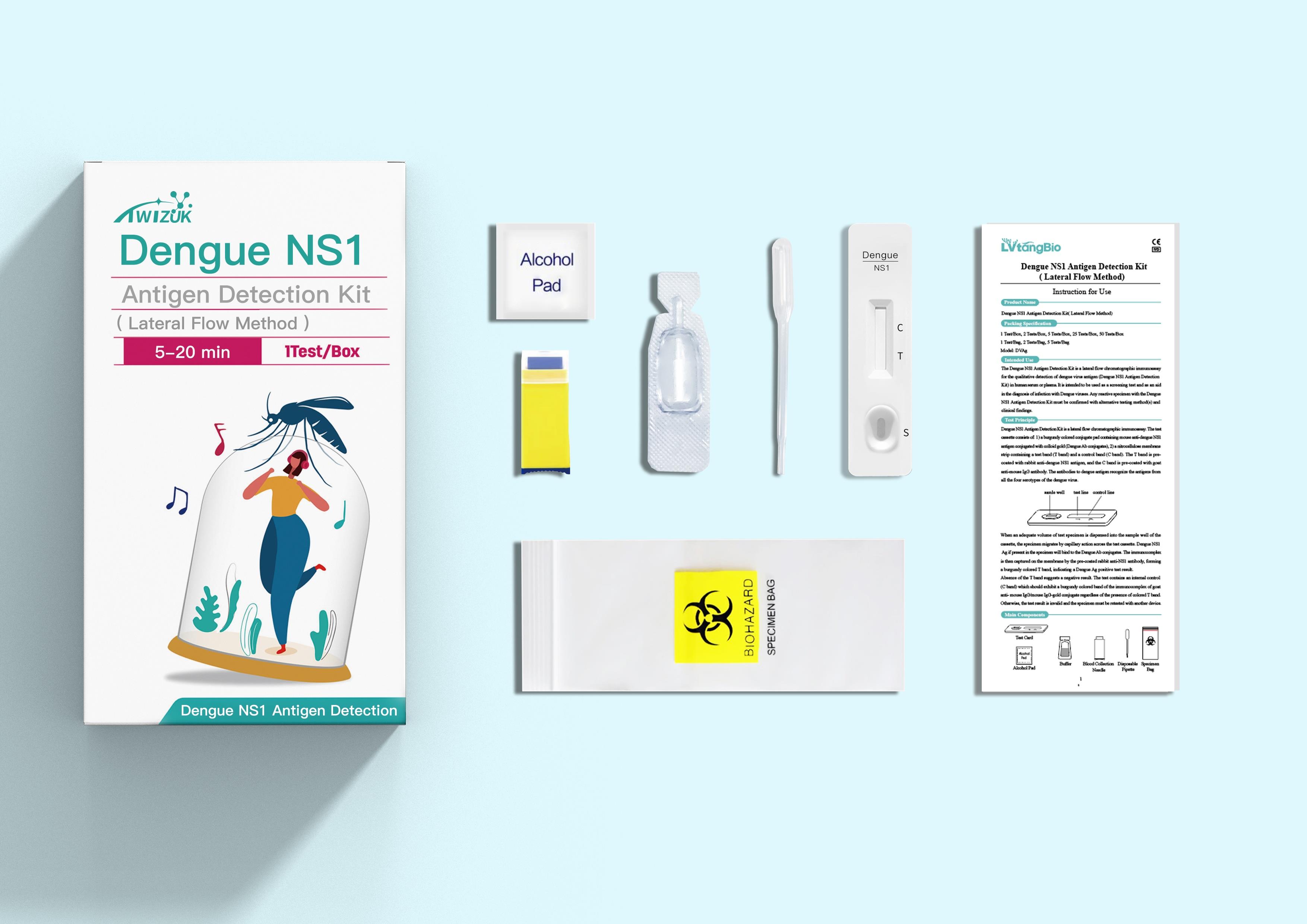
Brand : TWIZUK
Min.Order : 1000 Piece(s)

Brand : TWIZUK
Min.Order : 1000 Piece(s)

Brand : TWIZUK
Min.Order : 1000 Piece(s)
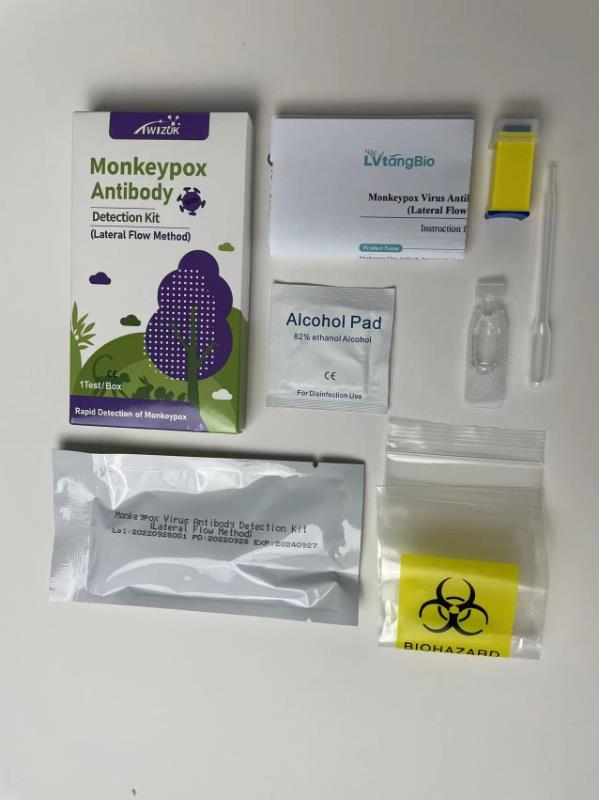
Brand : Twizuk
Min.Order : 1000 Piece(s)

Brand : Twizuk
Min.Order : 1000 Piece(s)
brand name : TWIZUK
certification :
fob price :
min.order : 1000 Piece(s)
warranty : \
payment terms : T/T
Packaing : 1 Test/Box, 5Tests/Box, 25Tests/Box
Specification : \
Trademark : TWIZUK
Production Capacity :
place of origin : Zhejiang
Manag Certifica : CE
Clinical sample |
COVID-19 Coronavirus Real Time PCR Kit (RT-PCR) | Total |
||
| Positive | Negative | |||
| SARS-CoV-2/Influenza A+B Antigen Detection Kit (N line) (Colloidal Gold Method) |
Positive | 145 | 0 | 145 |
| Negative | 3 | 517 | 520 | |
| Total | 148 | 517 | 665 | |
Clinical sample |
cobas® Liat® Influenza A/B & RSV Assay(RT-PCR) | Total |
||
| Positive | Negative | |||
| SARS-CoV-2/Influenza A+B Antigen Detection Kit (A line) |
Positive | 155 | 0 | 155 |
| Negative | 4 | 506 | 510 | |
| Total | 159 | 506 | 665 | |
Clinical sample |
cobas® Liat® Influenza A/B & RSV Assay(RT-PCR) | Total |
||
| Positive | Negative | |||
| SARS-CoV-2/Influenza A+B Antigen Detection Kit (B line) |
Positive | 144 | 1 | 145 |
| Negative | 5 | 515 | 520 | |
| Total | 149 | 516 | 665 | |