



FOB Price : Get a Price/Quote
Min.Order : 1000 Piece(s)
Certification : ISO13485,CE
Brand Name : Lumigenex
Payment Terms : T/T,EXW
brand name : Lumigenex
certification : ISO13485,CE
min.order : 1000 Piece(s)
warranty : 12months
payment terms : T/T,EXW
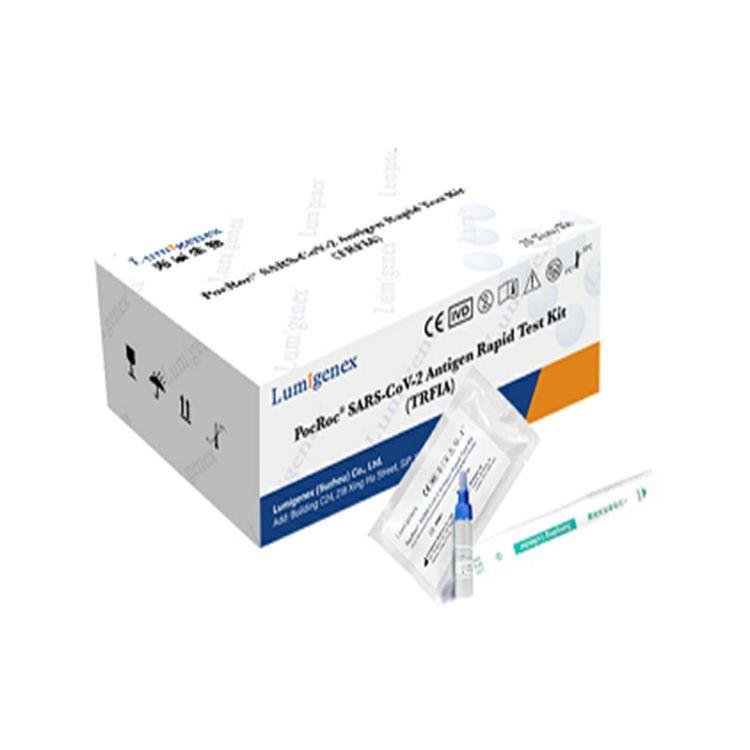
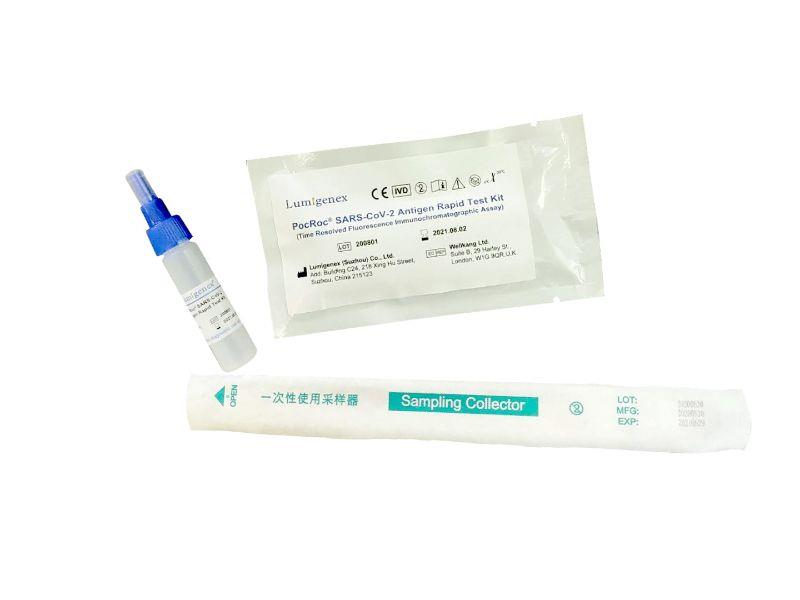
Packaging : 20Tests/Kit
Specification : 20Tests/Kit
place of origin : China

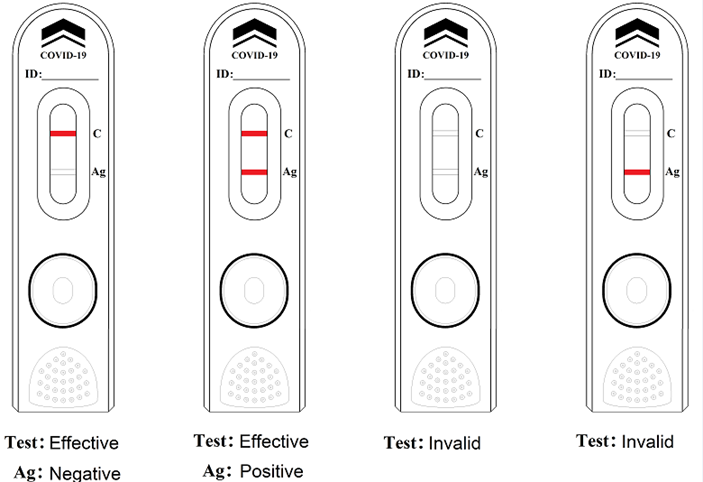
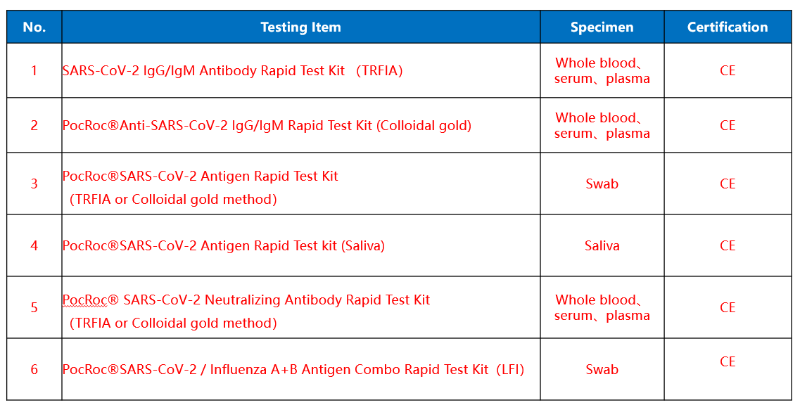


 Ordinary
Ordinary
 verified
verified
Business Type Service
Country / Region Jiangsu,China
Main Products POCT Diagnostic Reagents, Diagnostic Tests
Main Markets SouthAmerica,EasternEurope,SoutheastAsia,Africa,MidEast,WesternEurope

Brand : Lumigenex, PocRoc
Min.Order : 1000 Piece(s)
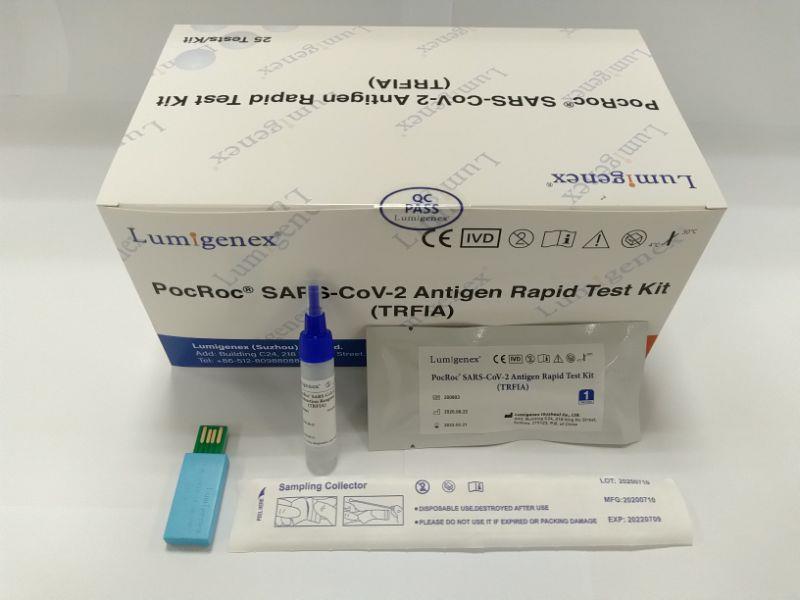
Brand : Lumigenex
Min.Order : 1000 Other(s)
Brand : Lumigenex
Min.Order : 1000 Piece(s)
Brand : Lumigenex
Min.Order : 1000 Piece(s)
Brand : Lumigenex
Min.Order : 1000 Piece(s)
brand name : Lumigenex
certification :
fob price :
min.order : 1000 Piece(s)
warranty : 12months
payment terms : T/T,EXW
Packaing : 20Tests/Kit
Specification : 20Tests/Kit
Trademark : Lumigenex
Production Capacity :
place of origin : China
Manag Certifica : ISO13485,CE


