trending topics
market reports
-

Registration Now Open: MEDICAL JAPAN 2026 OSAKA – Western Japan’s Largest Healthcare Trade Show
2026-02-10
-

MEDICAL JAPAN 2025 OSAKA Returns to Showcase Global Innovations
2025-02-17
-

Visit MEDICAL JAPAN 2023 TOKYO and take full advantage of the business opportunities!
2023-09-01
-
US to distribute 400 million free N95 masks at CVS, Walgreens in COVID fight
2022-01-21
-

Ethiopia receives additional 2.2 mln doses of Chinese-donated COVID-19 vaccines
2022-01-21
-
Hong Kong researchers say they develop novel material able to kill COVID-19 virus
2022-01-14
-

10 million more Chinese doses on way for Kenya
2022-01-14
-

Sino-African ties on track for a brighter future
2022-01-07
-
Efforts urged to boost COVID-19 vaccine production capacity in poor countries
2022-01-07
-
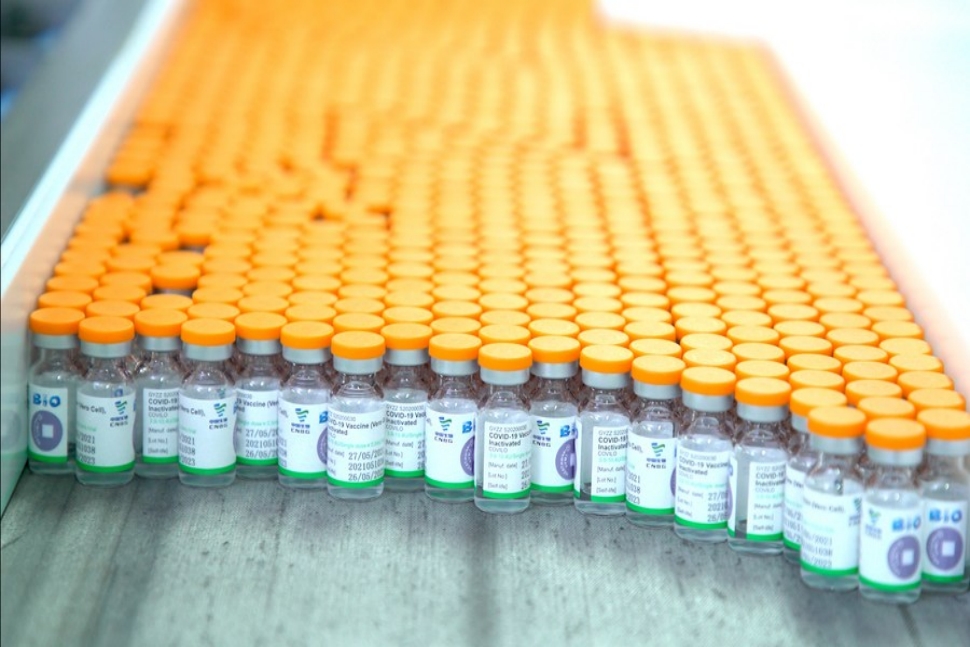
UAE approves Sinopharm's new protein-based COVID-19 vaccine
2022-01-07
Anti-depression drug fluvoxamine can prevent deterioration of mild COVID-19 infections
2020-12-16

A clinical trial conducted by researchers at Washington University in St. Louis of the US shows that fluvoxamine — an anti-depression drug — can prevent the deterioration of mild COVID-19 infections. However, more randomized and controlled studies on larger sample size are needed to verify the drug's clinical efficacy.
The research was published online by JAMA on November 12. It was a double-blind, randomized, completely remote clinical trial. The participants were 152 adults living in the community who were not hospitalized with COVID-19 infections.
None of the 80 infected subjects who received fluvoxamine treatment for 15 days experienced deterioration in their health, while six persons in the placebo group all suffered deterioration, according to the research.
However, due to the small sample size and short follow-up time, the conclusions of the study have certain limitations, the research noted. Therefore, a randomized, placebo-controlled trial with larger sample size is needed to verify fluvoxamine's clinical efficacy.
The infectious disease department of Huashan hospital in Shanghai, led by China's leading infectious disease specialist Zhang Wenhong, forwarded the research on its WeChat account on Tuesday and noted that other clinical trials involving the COVID-19 treatment efficacy of drugs such as Remdesivir and hydroxychloroquine have failed.
The research was published online by JAMA on November 12. It was a double-blind, randomized, completely remote clinical trial. The participants were 152 adults living in the community who were not hospitalized with COVID-19 infections.
None of the 80 infected subjects who received fluvoxamine treatment for 15 days experienced deterioration in their health, while six persons in the placebo group all suffered deterioration, according to the research.
However, due to the small sample size and short follow-up time, the conclusions of the study have certain limitations, the research noted. Therefore, a randomized, placebo-controlled trial with larger sample size is needed to verify fluvoxamine's clinical efficacy.
The infectious disease department of Huashan hospital in Shanghai, led by China's leading infectious disease specialist Zhang Wenhong, forwarded the research on its WeChat account on Tuesday and noted that other clinical trials involving the COVID-19 treatment efficacy of drugs such as Remdesivir and hydroxychloroquine have failed.
(Global Times)
label :



 My Member
My Member Message Center
Message Center