trending topics
market reports
-

MEDICAL JAPAN 2025 OSAKA Returns to Showcase Global Innovations
2025-02-17
-

Visit MEDICAL JAPAN 2023 TOKYO and take full advantage of the business opportunities!
2023-09-01
-
US to distribute 400 million free N95 masks at CVS, Walgreens in COVID fight
2022-01-21
-

Ethiopia receives additional 2.2 mln doses of Chinese-donated COVID-19 vaccines
2022-01-21
-
Hong Kong researchers say they develop novel material able to kill COVID-19 virus
2022-01-14
-

10 million more Chinese doses on way for Kenya
2022-01-14
-

Sino-African ties on track for a brighter future
2022-01-07
-
Efforts urged to boost COVID-19 vaccine production capacity in poor countries
2022-01-07
-
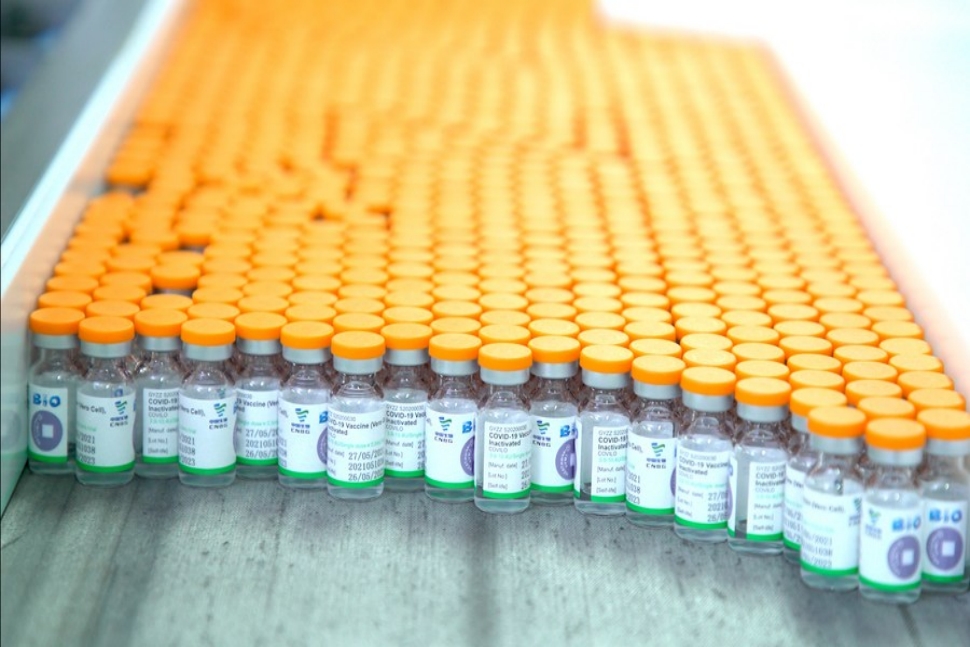
UAE approves Sinopharm's new protein-based COVID-19 vaccine
2022-01-07
-
UAE approves Sinopharm's new protein-based COVID-19 vaccine
2022-01-07
The 14th Healthy China Forum released the 14th Healthy China Forum · Top Ten medical devices
2022-08-30

Recently, the 14th Healthy China Forum, a comprehensive review of domestic listed medical devices, according to innovation, breakthrough, influence and other indicators, released the 14th Healthy China Forum · Ten medical devices. Among them, domestic medical devices account for half of the country. Al shadow medical
UMR Omega 3.0T magnetic resonance imaging system: uMR Omega is the world's first 75cm ultra-large aperture 3.0T magnetic resonance. According to the director of United Imaging Medical, this magnetic resonance has the largest aperture in the industry, which expands the space by 25% compared with the traditional equipment. It can provide spacious space and comfortable experience for the patient, especially for the benefit of large patients, pregnant women, claustrophobic patients and other groups, and also ensure high-quality imaging.Medtronic MAZOR X Intelligent navigation robot for spinal surgery, as the world's first spinal surgery robot equipped with Medtronic's advanced navigation technology to achieve full visualization, MAZOR X brings new clinical ideas, compared with traditional spinal surgery, MAZOR can X for clinical doctors to provide intelligent preoperative planning, intraoperative under precise positioning, navigation, mechanical arm embedded tool and visible in the whole process of the implant, to break through the traditional blind area in a limited field of vision, such as the bottleneck, accomplish truly "brain", "hand", "eye" coordination, achieve results foreseeable operation effect, effectively reduce intraoperative bleeding and operation time, Improve surgical safety and accuracy.Boston Scientific
AXIOS cutting drainage stent, AXIOS cutting drainage stent is the world's first for intracavitary treatment stents guided by endoscopic ultrasonography, from a diagnostic and therapeutic endoscopic ultrasonography. Clinically, pancreatic pseudocysts and encapsulated necrosis are common complications in patients with acute pancreatitis.
Compared with traditional surgery, Axios stent, as a revolutionary endoscopic intervention solution, provides patients with a less invasive and more convenient treatment option, and the surgical process is simple, which can be carried out by most endoscopic centers. After appearing on the new product release platform of CIIE last year, the product has been widely concerned by the industry, and has been officially approved by the National Medical Products Administration for marketing.Siemens Medical MAGNETOM Lumina Holographic 3T Magnetic Resonance, the Lumina 3T is equipped with GO 3D whole-body clinical solutions, enabling one-click accelerated 3D clinical solutions, based on the latest Siemens Medical Magnetic Resonance technology platform. It is expected to fundamentally change the way of routine MRI scanning and further aid clinical decision making.Shanghai Minimally invasive Dragonfly Eye DFVision 3D electronic abdominal endoscope, Shanghai minimally invasive Dragonfly eye DFVision 3D electronic abdominal endoscope。Carl Zeiss fundus Camera CLARUS 500, the Zeiss CLARUS 500 can perform retinal image analysis without pupil dilation, avoiding blurred vision and light sensitivity caused by the use of pupil dilation agents, making the imaging process safer and more comfortable, and avoiding the need for patients to wait 30 minutes before fundus examination for pupil dilation. The diagnosis and treatment process has been comprehensively optimized. By moving the optical lens toward the patient during the examination, the Zeiss CLARUS 500 image is not obscured by the eyelashes of the eyelid, which is very conducive to the observation of peripheral retinopathy, reducing repeated photography and creating a comfortable and satisfactory examination experience for the patient.Suzhou Tongxin Medical Magnetic-Flux implantable left ventricular assist System (LVAD) is the first domestic "artificial heart" that has successfully completed 50 clinical trials and the largest number of implants in China according to the regulations of the National Food and Drug Administration.
Compared with the widely used maglev VAD, Cifu VAD has reached the international leading level in the key performance indicators of VAD evaluation, such as blood compatibility, implant invasion, and anti-infectivity. Tongxin Medical has become the benchmark for the development of technology in the industry, and has gained the international reputation as a VAD technology breakthrough.Suzhou Tongxin Medical Magnetic-Flux implantable left ventricular assist System (LVAD) is the first domestic "artificial heart" that has successfully completed 50 clinical trials and the largest number of implants in China according to the regulations of the National Food and Drug Administration.
Compared with the widely used maglev VAD, Cifu VAD has reached the international leading level in the key performance indicators of VAD evaluation, such as blood compatibility, implant invasion, and anti-infectivity. Tongxin Medical has become the benchmark for the development of technology in the industry, and has gained the international reputation as a VAD technology breakthrough.Shandong weigao wonderful hand surgery robot, "wonderful hand" robot has the characteristics of miniaturization and integration, the layout optimization, structure compact, already broke through the minimally invasive surgery wire multi degree of freedom mechanical transmission of decoupling design, from the operator the reconfigurable layout principle and implementation of variants isomorphism, system control model to build three key technical problems, such as solving the robot complete sets of technical problems. At present, the robot system has applied for more than 60 invention patents, of which more than 40 have been authorized.Shenzhen Xianjian Xinkang 8301 temporary pacemaker, the 8301 temporary pacemaker independently developed by Xianjian Technology was approved by FDA and CE in 2019 and the first half of 2020. In China, it entered the "green channel" in 2019 and was approved for market in April 2021.
The product is a two-in-one pacing/analysis multifunctional product designed to provide single-chamber external temporary pacing for patients with bradycardia, as well as for pacing system analysis during pacemaker and defibrillator implantation procedures. In addition, the device can continuously measure the impedance and sensing amplitude of the pacing lead system, and continuously display the intraluminal ECG in real time, which is suitable for the clinical observation of the injury current after active electrode lead implantation.Yak Wisdom "Yak wisdom" tooth planting robot, Yak wisdom tooth planting robot is the first dental implant robot with independent intellectual property rights in China. The research and development team comprehensively integrated intelligent robots, machine vision, multi-sensor information fusion, three-dimensional graphics visualization and other top technologies, and solved the key problems of minimally invasive surgery in the narrow non-direct vision space of the mouth in 10 years, significantly improving the surgical accuracy, safety and efficiency of implant teeth.
In October 2020, the world's first autonomous oral implant robot, jointly developed by Academician Zhao Yimin and his team, Wang Lifeng and others, and developed by Yake Wisdom, won the green approval channel for innovative medical devices from the National Food and Drug Administration.
label :



 My Member
My Member Message Center
Message Center